
Note: Faraday's law of electrolysis: The laws state that (1) the amount of chemical change produced by current at an electrode-electrolyte boundary is proportional to the quantity of electricity used, and (2) the amounts of chemical changes produced by the same quantity of electricity in different substances are proportional to their equivalent weights. When the reaction comes to equilibrium, what is the cell voltage A) 0.43 V B) 1.11 V C) 0.78 V D) -0. What happened at the other electrode (A) 0.05 mole of Cu2+ ions passed into solution (B) 0. Study with Quizlet and memorize flashcards containing terms like Consider the following reaction: 2Fe2+(aq) + Cu2+ 2Fe3+(aq) + Cu. A dilute solution of copperIIchloride containsCu2ionsClionsHions andOHionsOn electrolysis it givescopper at the cathode as copper is less reactive than. Write the reduction half-reactions that turn the neutral element into its ion by adding electron(s). In the electrolysis of CuCl2 solution using Cu electrodes the mass of cathode increases by 3.18g. In the electrolysis of molten copper chloride, the substance liberated at the anode is chlorine and the substance deposited at cathode is copper. Summary: Determine what ions came from the compound.

These are known as Faraday's laws of electrolysis.
CATHODE REACTION IN ELECTROLYTSIS OF CUCL2 SERIES
The quantity of the products is proportional to the current, and when two or more electrolytic cells are connected in series to the same power source, the products produced in the cells are proportional to their equivalent weight. The voltage that is needed for electrolysis to occur is called the decomposition potential. Solution: due to electrolysis of calcium chloride, first this compound converts into ions Ca² and Br ¯. (3) overall cell reaction for this process. (2) half reaction that takes place at the cathode. To find: (1) half reaction that takes place at the anode. This is the decomposition/electrolysis reaction: 2H2O2H2+O2 and this is the. Given info: The electrolysis of molten calcium chloride produces calcium and chlorine. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. Consider the following reactions with HI in. Negatively charged ions (anions) move towards the electron-extracting (positive) anode.Įlectrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction.
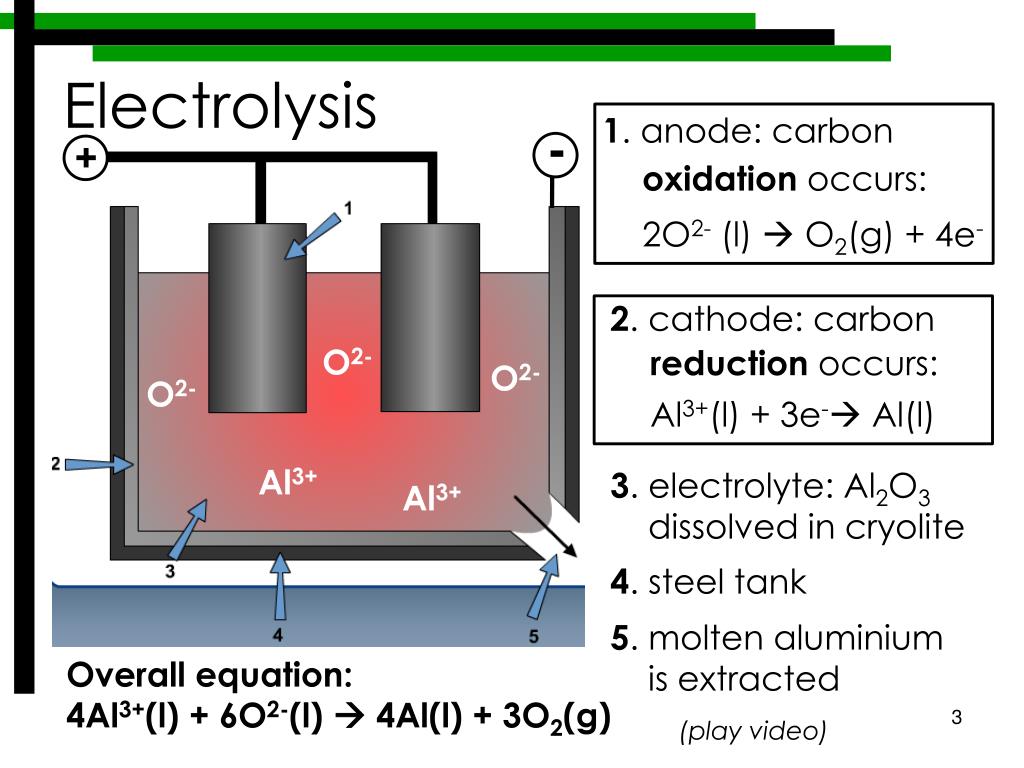
Positively charged ions (cations) move towards the electron-providing (negative) cathode. Hint: Each electrode attracts ions that are of the opposite charge.


 0 kommentar(er)
0 kommentar(er)
